Medical network - December 19 December 16. CFDA website issued a "about three clinical trials for medical device registration project supervision and spot check on the announcement. Announcement, 10, 2016 to November, the CFDA group has carried out the second batch of medical instrument clinical trial selectives examination, examination revealed three for medical device registration project authenticity problems in clinical trials. Respectively is:
Anhui equivalent biological technology co., LTD., the human papilloma virus (15) nucleic acid parting detection kit (PCR multicolor fluorescence) (accepted number: CSZ1600248), Germany Roche Diagnostics GmbH T lymphocyte virus type 1 and type 2 antibody detection kit (electrochemical luminescence method) (accepted number: JSZ1600012) and Japan LSI Medience Corporation soluble CD14 subtype detection kit (chemiluminescence immunoassay) (accepted number: JSZ1600078) three registration project sample reuse, exist in the process of clinical trials and clinical trial scheme design samples not repeat use, test report on the sample reuse without special description.
In accordance with the relevant provisions of the medical device registration, the CFDA authenticity problems of the above three registration items shall not be registered, from will not be registered again within one year from the date of the will not be accepted, involving the clinical test units and the relevant requirements of the relevant provincial food and drug administration in accordance with the relevant provisions of the regulations on the supervision and administration of medical devices to investigate, and report to the administration of the processing results.
In addition, the CFDA website also released on the released 27 companies withdraw 32 medical instrument announcement of registration of the project. On October 20th - on December 12, a total of 27 companies withdraw 32 for medical device registration project.
Has withdrawn his application for medical device registration project list
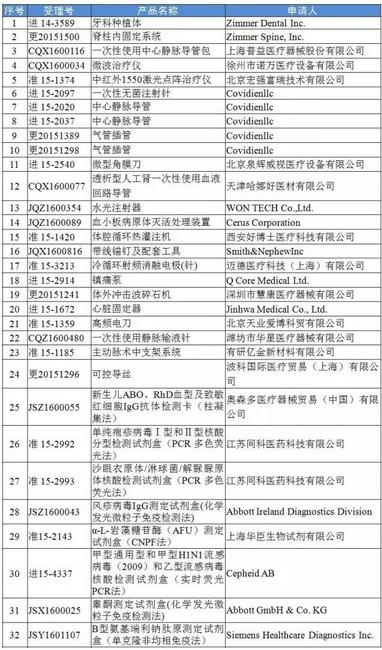
|
