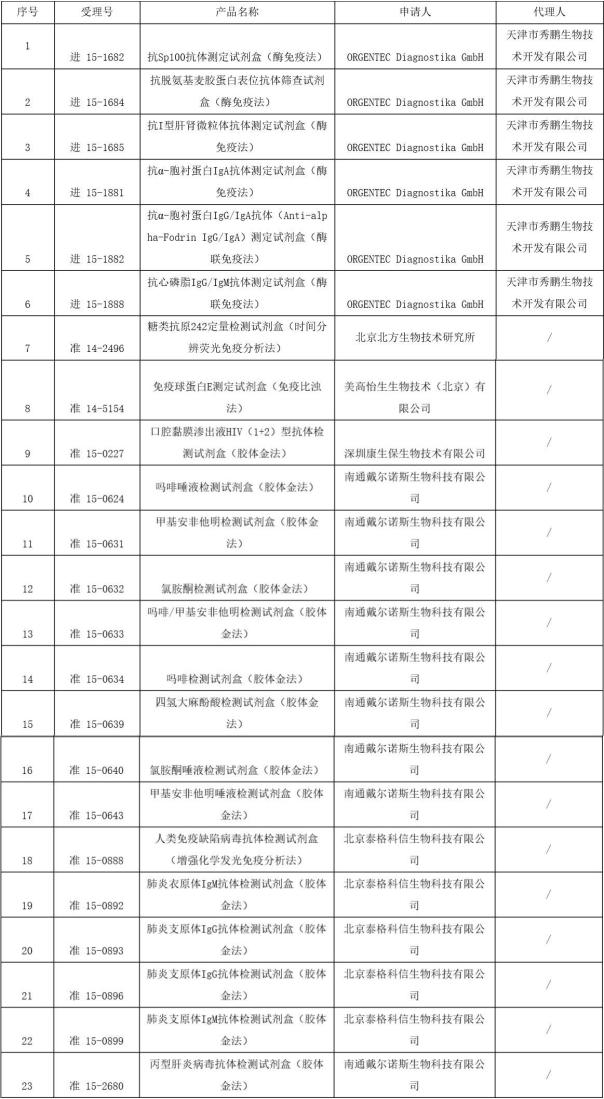
Medical network - on October 19, 17, total bureau of national food and drug supervision and administration of administrative processing service center issued a notice of approval documents on October the first batch of medical devices shall not be registered, the involved 23 products, whether it's domestic or imported for the in vitro diagnostic products. Specific details are as follows:
|
