Medical network - December 21, 2016 is coming to an end, for now, the number of new molecular entities drugs approved by FDA than in previous years has been showing a sharp decline trend. The problem: in the past two years, the research and development of pharmaceutical industry's amazing productivity is only a flash in the pan?
If the pharmaceutical industry innovation output forecast proved unable to keep high historical value, in spite of reduced moderately favorable dry drug research and development of sustainable development, but the confidence of investors will hit.
Up to 25 new drug approvals
If Roche (Roche) Ocrevus Solithera smoothly with Cempra company at the end of December, so in 2016 the FDA approval of new molecular drug and biological products will be only 25.
Roche's resistance to multiple sclerosis drug Ocrevus may bring the huge commercial value made it to one of the most popular focus this year. Who reported for the drug have previously in accordance with the prescribed fee act (PDUFA))) submitted to the FDA reported for the corresponding cost to improve the efficiency of the review, the FDA has said preliminary results will be announced on December 28th the review, it is not through the review of the possibility is very small.
Big ring, by contrast, the lipid antimicrobial Solithera encountered security problems is significant, though its in the fight against some very dangerous bacterial infection of the importance of it is indisputable, but the result of examination and approval is still difficult to determine. Oral and injected the drug dosage form of PDUFA review results will be released on December 27, 28, respectively.
However, may suffer from not just Cempra refused to company. Many to catch in 2016 has received FDA approved drugs complete reply, EP Vantage has yet to do quantitative analysis on the number of reported for new drugs, from the point of existing, such as data and phenomenon, the FDA seems to accept a lot this year file center daily news.
Also not through risk exists in the review of the senor not sarilumab (Sanofi), its earlier in November, has received the delay notice, the reason is that there are some production issues. The rheumatoid arthritis drug is one of major hotspot that 2017 new drugs, some worry that the Sarilumab approved risk may also affect the regeneration yuan (Regeneron) and senor of joint research and development of drug allergy test dupilumab review, which is analyst forecasts for a greater market potential, the review results will not be released in March next year. If the two medicine cannot be approved smoothly, senor will face greater market pressure.
New business value
Opdivo Harvoni and Ibrance it listed a few in the five years since 2014201 the success of the blockbuster drug model in the future for a long time, I'm afraid it is difficult to be copied. Analysts believe that, in recent years, both has the huge commercial value in listing 5 years of sales by the number of new drugs listed, or the absolute number of new drug approvals, than the average over the past 10 years.
Although Ocrevus and Solithera receiving FDA approval, also can't change the 2016 new drug approval number is low, in the past ten years the results of the average. 2016 new business value level, however, will not be affected by too big, 5 years sales forecast and near 10-year average. Some potential blockbuster drugs have been approved this year, such as Tecentriq Epclusa and Venclexta, it will lead to sales of comparable size.
, of course, the output of the pharmaceutical industry innovation drugs only is one indicator of measuring the level of productivity, there are plenty of reasons to think that on the analysis of the indicators should be presented separately. For example, such analysis is not the time cost of new drug research and development and the index of capital into consideration; For the wide application of these drugs for various diseases related to data is only a conservative to open, so the index is not suitable for together with other indicators included in the comprehensive evaluation.
All that said, although in 2016 the number of new drugs approved back to 2007 levels, but the good news is that in 2016 approved the new drugs have more commercial value is expected to improve.
In 2017, the pharmaceutical industry innovation whether drug approval number will shrink is still unknown. In spite of 2014201 years of blowout growth can't maintain for a long time, but the pharmaceutical industry should focus on consolidating since 2010 presents the momentum of development of productivity, to prevent it in the next few years down the momentum of the current fall down first a stagnation of 10 years in the intermediate stage of development level, that for dry pharmaceutical industry will be a big disaster. (medicine economic news Wu Yingyi)
Editor's remit
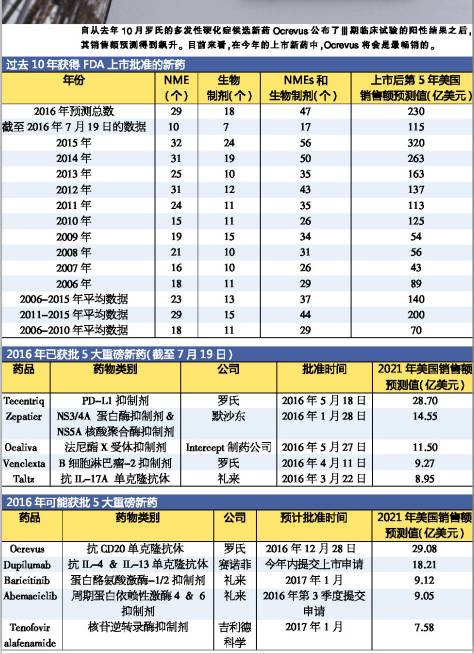
2016 EP Vantage analysis during the first half of this year has been listed and approved new molecular medicine (NMEs) and biological agents, and forecasts its market sales in the United States five years later, and lists the top five new drugs.
Roche r&d Tecengtrip is first used in the treatment of bladder cancer PD - L1 inhibitor, in May this year. MSD (Merck) of hepatitis c drug Zepatier at this year's approval of new drugs is still among the highest in 5 years sales forecast, and Intercept of the company's drug treatment of primary biliary cirrhosis Ocaliva ranked third in the list.
In has not yet been approved by the drug have not filing Sanofi (Sanofi) dermatitis medicine dupilumab and lilly's breast cancer drug resistance abemaciclib. Both the breakthrough treatments that, but the review result will not be released in March next year.
The other two drugs are lilly (EliLilly) in rheumatoid arthritis drug baricitinib and gilead tenofoviralafenamide (GileadSciences) of hepatitis b treatment drugs, the review deadline is in January next year, they are likely to get the authorisation in advance.
(source:EP Vantage) |
